More Reports
|
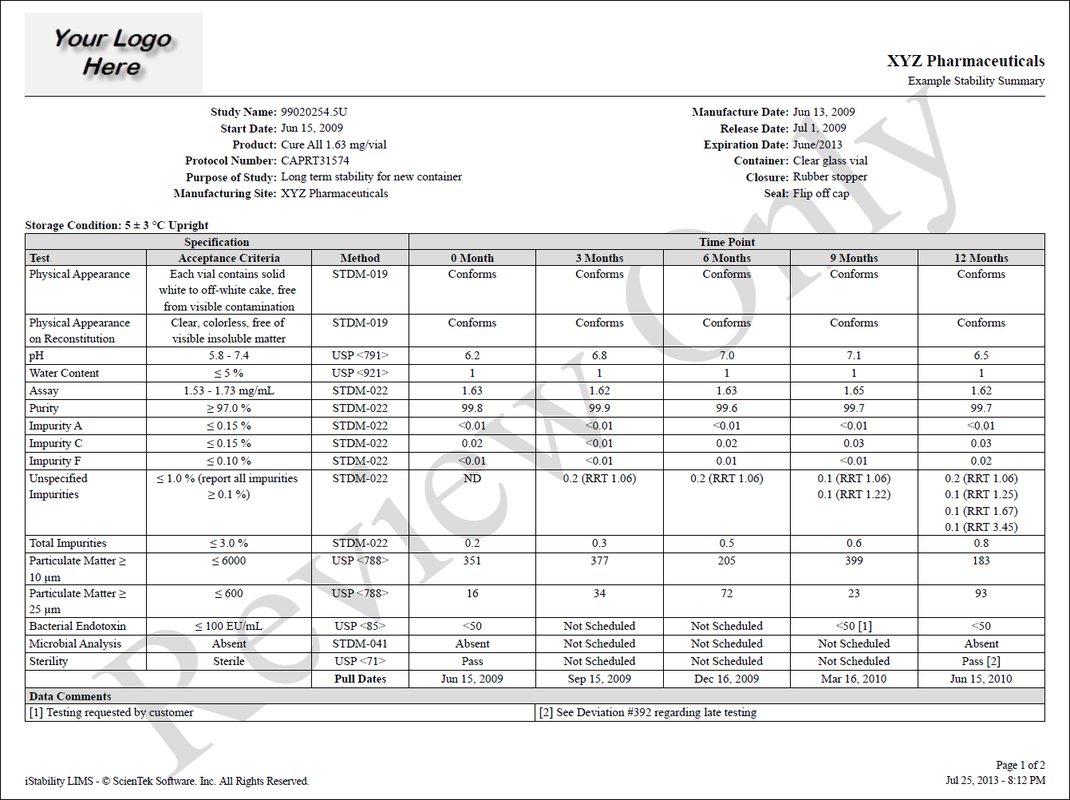
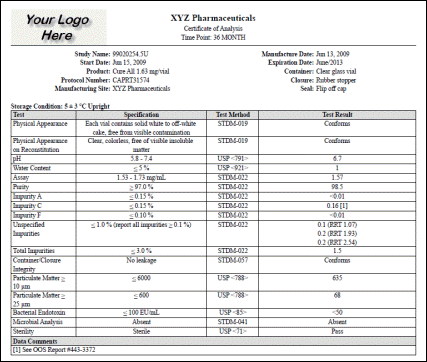
Study Summary
Your entire study in one report. All study ID, notes, testing schedules, sample history, test specifications, and test results in one easy to understand report. |
OOS Data
Quickly pull up a list of out-of-specification data for selected studies, complete with any associated comments. Be ready for any regulatory or customer inspection. |
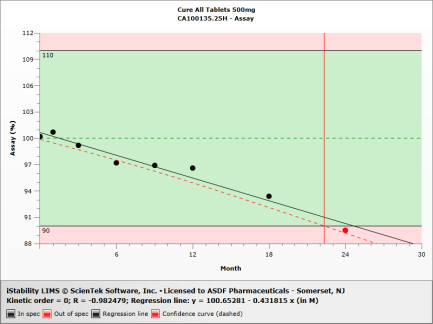
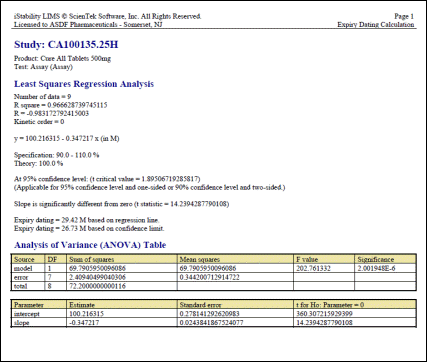
One Test Table
Easily compare data for one test across many studies for one product. Allows for comparisons between different lots, packaging configurations, and manufacturers. |
|
Workload Schedule
Have your upcoming testing schedule automatically emailed to your inbox on a regular schedule that you specify. Pull up your workload instantly from within iStability LIMS. |
Overdue Tests
Never let a test become overdue with periodic notifications of tests that will soon become overdue unless addressed. Powerful management of tests with test windows that can be tailored to any stability design. |
Blank Report Forms
iStability LIMS is designed so that laboratory personnel can directly enter data into its database. However, sometimes things are easier with hardcopy, blank report forms (think of these as test request forms). |